Get the free "Lewis structure" widget for your website, blog, Wordpress, Blogger, or iGoogle Find more Chemistry widgets in WolframAlphaWrite the Lewis structure for the guanadinium ion, C(NH 2) 3 1, and include all relevant resonance forms (Note that the C is bonded to three N atoms) Note that you do not need to indicate FC for this problem, but you should always consider FC when writing Lewis structures 5 The skeletal structures of two amino acids, leucine and = 145 = 10 valence electrons Hence, Hydrogen Cyanide, HCN, has ten valence electrons HCN Lewis structure Once you get the total number of valence electrons, you can make a Lewis dot structure of HCN This structure helps in understanding the arrangement of valence electrons around the atoms in the molecule
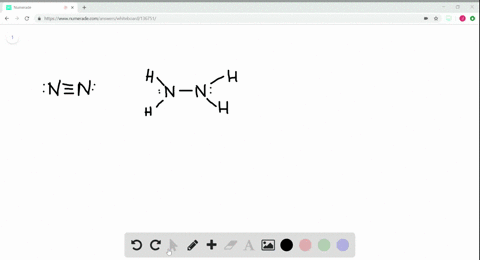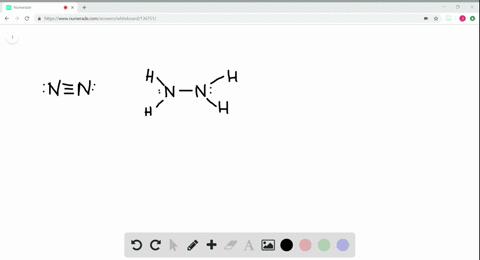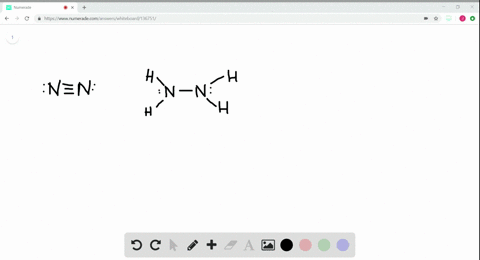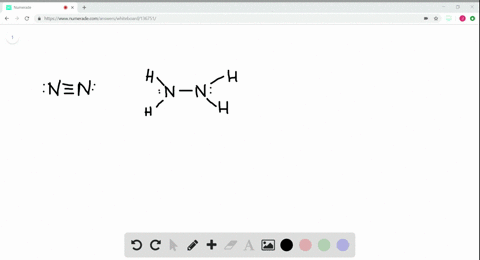
Solved A Draw Lewis Structures For Ethane Left Mathrm C 2 Mathrm H 6 Right Ethylene Left Mathrm C 2 Mathrm H 4 Right And Acetylene Left Mathrm C 2 Mathrm H 2 Right B What Is The Hybridization Of The Carbon Atoms In
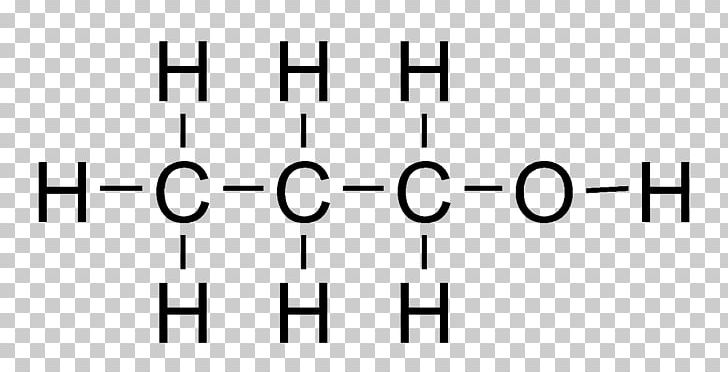
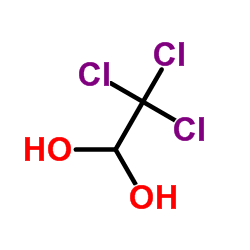
1 1-ethanediol lewis structure
1 1-ethanediol lewis structure-1b) 2 BP and one LP, VSEPR notation AX 2E1 • the two BP are pushed closer together by the lone pair • the X – A – X bond angle isStructure, properties, spectra, suppliers and links for 1,2Ethenediol



1 Propanol Lewis Structure Structural Formula Butanol Png Clipart 2butanol Alcohol Angle Area Black Free Png
Ethylene glycol is produced from ethylene (ethene), via the intermediate ethylene oxide Ethylene oxide reacts with water to produce ethylene glycol according to the chemical equation C 2 H 4 O H 2 O → HO−CH 2 CH 2 −OH This reaction can be catalyzed by either acids or bases, or can occur at neutral pH under elevated temperaturesSubstance identity The 'Substance identity' section is calculated from substance identification information from all ECHA databases The substance identifiers displayed in the InfoCard are the best available substance name, EC number, CAS number and/or the molecular and structural formulas Some substance identifiers may have been claimedStructure, properties, spectra, suppliers and links for 1,1Ethanediol,
In the Lewis structures listed here, M and X represent various elements in the third period of the periodic table Write the formula of each compound using the chemical symbols of each element (a) (b) (c) (d) Write the Lewis structure for the diatomic molecule P 2, an unstable form of phosphorus found in hightemperature phosphorus vaporChemical structure This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript Species with the same structure Pluronic f681 Reaction with Acids Cyclic sulfites and cyclic sulfates of ethanediol undergo hydrolysis with acids to furnish glycol The substituted cyclic sulfate, such as tetramethyl1,3,2dioxathiolane 2dioxide, may undergo a pinacol type of rearrangement under acidic conditions to furnish pinacolone in good yield (74JOC3415)
1,2Ethanediol, acetate The 'Substance identity' section is calculated from substance identification information from all ECHA databases The substance identifiers displayed in the InfoCard are the best available substance name, EC number, CAS number and/or the molecular and structural formulas Some substance identifiers may have beenLewis base adducts of lead(i1) compounds 111* synthesis and structural characterization of lpb(c10,), and lpb(ncs),, (l= meso5,7,7,12,14, 14hexamethyl 1,4,8,11 tetraazacy cotetradecane) Aims To purify and characterize the (R)specific carbonyl reductase from Candida parapsilosis;




Solved A Draw Lewis Structures For Ethane Left Mathrm C 2 Mathrm H 6 Right Ethylene Left Mathrm C 2 Mathrm H 4 Right And Acetylene Left Mathrm C 2 Mathrm H 2 Right B What Is The Hybridization Of The Carbon Atoms In




Emulsifiers Smoothex G M S Glyceryl Mono Stearate प यस क र Fine Zeelandia Private Limited Mumbai Id
Ethane1,2diol, (ethylene glycol, monoethylene glycol, MEG) which is manufactured from ethene via epoxyethane, is used to make polyester fibres, resins and films although it is probably better known for its use as a coolant in cars It is miscible with water and it lowers the freezing point of water so it is used as an antifreezeEnglish Structure of 1,1ethanediol Date March 09 Source Own work Author Leyo Public domain Public domain false false This image of a simple structural formula is ineligible for copyright and therefore in the public domain, because it consists entirely of information that is common property and contains no original authorshipTo compare the enzyme with other stereospecific oxidoreductases;
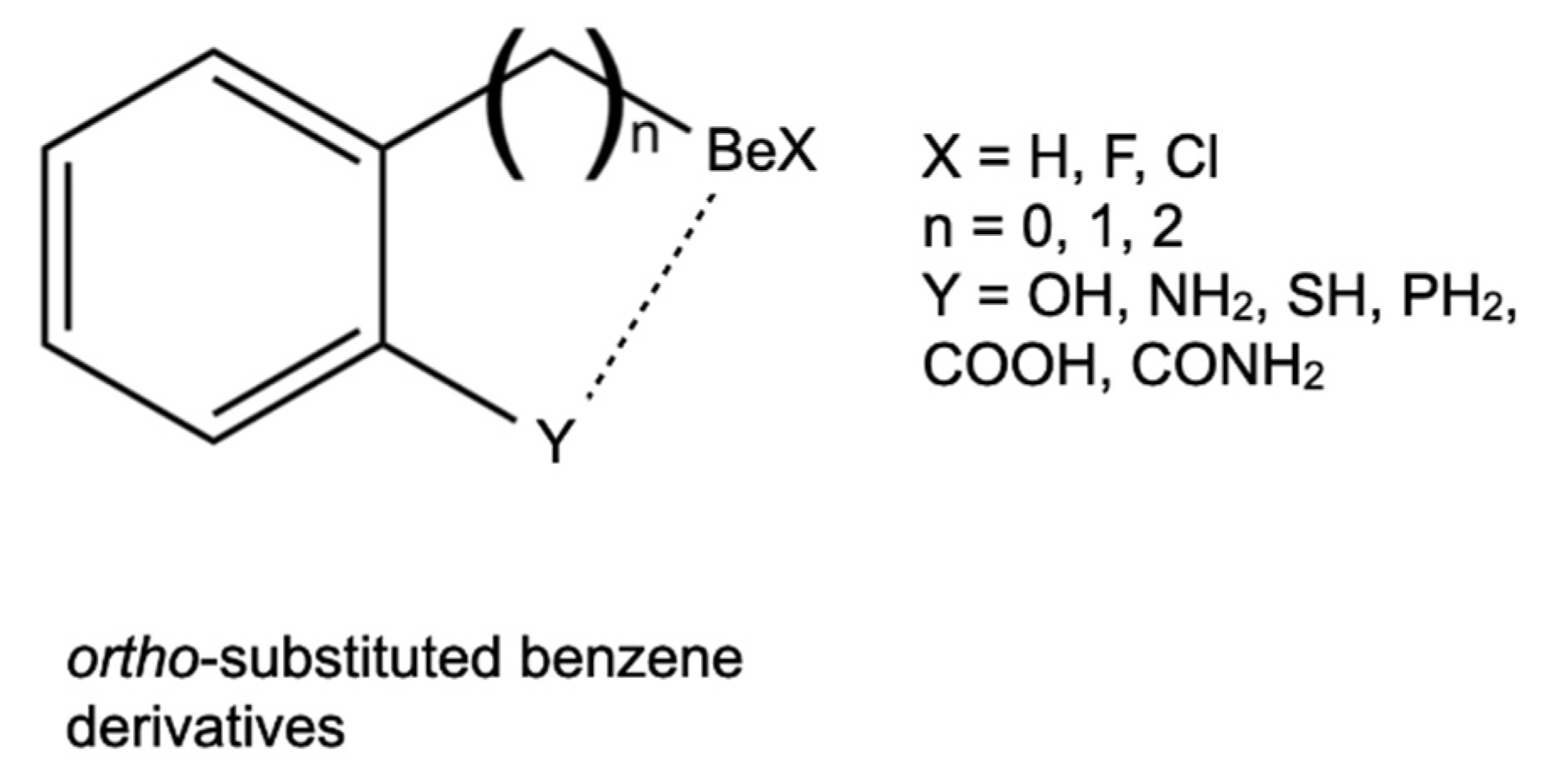



Molecules Free Full Text Large Stabilization Effects By Intramolecular Beryllium Bonds In Ortho Benzene Derivatives Html
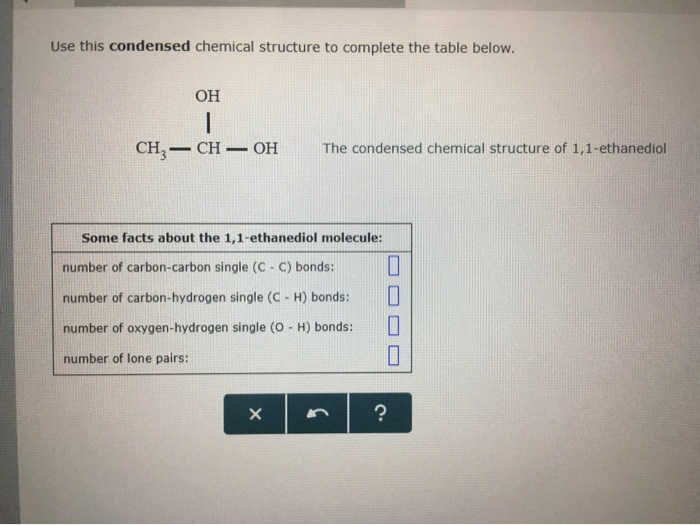



Solved Use This Condensed Chemical Structure To Complete The Chegg Com
Structural Formula C 2 H 6 S ethanethiolAnswer and Explanation 1 The Lewis structure of CON − − is shown in the diagram below The total number of valence electrons for CON − − is the sum of the valence electrons of carbonEthylene glycol is a 1,2glycol compound produced via reaction of ethylene oxide with water It has a role as a metabolite, a toxin, a solvent and a mouse metabolite It is a glycol and an ethanediol




Interpreting Condensed Chemical Structures Aleksmeaghane Troy4 25 Meaghane Troy4 25 pmest Course Hero




1 2 Ethanediol Structure Shefalitayal
A P with 5 dots surrounding it Based on your electron configuration, the element that you want to draw is Phosphorus, since you have 15 electrons Phosphorus has 5 valence electrons To draw Lewis Structures for elements, the symbol for the element is drawn with the number of valence electrons it has surrounding it So, to draw the Lewis Structure, begin by drawing the on Lewis Dot Structure Worksheet 1 Answer Key Nh4 Lewis Structure How To Draw The Dot Structure For Nh4 Ammonium Science Chemistry Molecular Geometry Chemistry Worksheet Elements And Compounds 1 Teaching Chemistry Matter Science Chemistry Lessons Chiral and Achiral compound Chiral Compound An asymmetric carbon atom (chiral carbon) is a carbon atom that is attached to four different types of atoms or groups of atoms, and the Compound called Chiral compound Properties of Chiral carbon 1 Chiral compound do not superimpose 2 Plane of symmetry absent (so called asymmetric carbon) 3 Are optically



C2h6o2 Lewis Structure
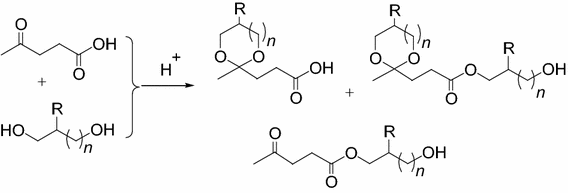



Acid Catalyzed Competitive Esterification And Ketalization Of Levulinic Acid With 1 2 And 1 3 Diols The Effect Of Heterogeneous And Homogeneous Catalysts Springerlink
B 1, 2ethanediol c 5methyltrans2heptene Structures of Organic Compounds The structures of organic compounds can be represented as either Lewis structures,Question 1 Draw the best Lewis structure, and resonance contributors of equal energy (if any), for the molecule C10, Answer the following questions based on your Lewis structure(s) a) How many valence electrons are in your Lewis drawing?Lewis Structures Lewis structures are used to help visualize what molecules look like They are 2dimansional representations of molecular structures, based on the arrangement of valence electrons in the formation of chemical bonds It is assumed that most elements require an octet of valence electrons, to attain a noble gas configuration There are exceptions to this rule, which we




Organic Chemistry I Homework 1998




4 Build A Model Of The 1 2 Ethanediol Molecule A Identify The Electron Pair And Molecular Geometry Around Homeworklib
Submit Antwer Tries 0/99 D) Are formal charges present in any of your structures?Drawing Lewis Structures (1) • Step 1Determine the total number of valence electrons for the molecule or polyatomic ion (These are the electrons that will be represented in your Lewis structure by lines for twoelectron bonds and pairs of dots for the lone pairs)Example Write the Lewis structure for the ammonium ion (NH 4 ) Answer Hydrogen atoms are always placed on the outside of the molecule, so nitrogen should be the central atom After counting the valence electrons, we have a total of 9 5 from nitrogen




Dna Interaction And Anticancer Evaluation Of New Palladium Ii Platinum Ii And Silver I Complexes Based On D And L 1 2 Bis 1h Benzimidazol 2 Yl 1 2 Ethanediol Enantiomers Sciencedirect



1 2 Ethanediol
CAS Registry Number ;In this example, we can draw two Lewis structures that are energetically equivalent to each other — that is, they have the same types of bonds, and the same types of formal charges on all of the structuresBoth structures (2 and 3) must be used to represent the molecule's structureThe actual molecule is an average of structures 2 and 3, which are called resonance structures 87qSiU0TC InChI InChI=1S/C6H10O4/c14 (7)96 (3)105 (2)8/h6H,13H3 InChIKey ACKALUBLCWJVNBUHFFFAOYSAN Google Search Mol Weight g/mol Molecular Formula




98 7 Ethyl 2 3ar 4s 6r 6as 6 Amino 2 2 Dimethyltetrahydro 3ah Cyclopenta D 1 3 Dioxol 4 Yl Oxy Acetate Oxalate C H No C H O Trc




Draw Lewis Structures For Ethane C 2h 6 Ethylene Quizlet
I quickly take you through how to draw the Lewis Structure of CH3CH2OH (Ethanol) I also go over hybridization, shape, sigma, pi bonding and bond anglesDrawing the Lewis structure for C 2 H 4 (named ethene) requires the use of a double bond In a double bond two pairs of valence electrons are shared (for a total of four valence electrons) Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shellCambridge Structural Database, version 531 and addenda up to 26 February 10) yielded 12 hits with structures determined with R val



Staggered Vs Eclipsed Conformations Of Ethane Master Organic Chem



1
1,4Dioxane (/ d aɪ ˈ ɒ k s eɪ n /) is a heterocyclic organic compound, classified as an etherIt is a colorless liquid with a faint sweet odor similar to that of diethyl etherThe compound is often called simply dioxane because the other dioxane isomers (1,2and 1,3) are rarely encountered Dioxane is used as a solvent for a variety of practical applications as well as in the laboratoryAnd to develop an available procedure producing optically active (R)1phenyl1,2ethanediol (PED) Methods and Results An (R)specific carbonyl reductase was found and purified from C parapsilosis through Lewis Structure Examples The Lewis electron dot structures of a few molecules are illustrated in this subsection 1 Lewis Structure of CO2 The central atom of this molecule is carbon Oxygen contains 6 valence electrons which form 2 lone pairs Since it is bonded to only one carbon atom, it must form a double bond




1 1 Ethanediol Semantic Scholar




Propane 1 2 Diol An Overview Sciencedirect Topics
The distance C7C8 ( (19) Å) corresponds well to the pertinent distances previously observed in well determined structures with the indoline2,3dione fragment The search in the Cambridge Structural Database (Allen, 02;1,2Ethanediol HOCH2CH2OH or CH2OHCH2OH or C2H6O2 CID 174 structure, chemical names, physical and chemical properties, classification, patents, literatureEthane1,2diol chemical information, properties, structures, articles, patents and more chemical data



Solved 1 Write The Three Staggered And Three Newman Projections For 2 3 Dimethylhexane Looking Down The C3 C4 Bond Rank All Six From Most 1 Course Hero




New Set Of Multicomponent Crystals As Efficient Heterogeneous Catalysts For The Synthesis Of Cyclic Carbonates Abstract Europe Pmc
1,1Ethanediol C2H6O2 CID structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety Lewis dot structures can be drawn to show the valence electrons that surround an atom itself This type of Lewis dot structure is represented by an atomic symbol and a series of dots See the following examples for how to draw Lewis dot structures for common atoms involved in covalent bonding Example 1 Draw the Lewis Dot Structure for theLewis structures don't tell us everything, but along with molecule geometry and polarity they are hugely informative Search 100 Lewis Structures on our site (Opens new window) Click the Chemical Formula to see the Lewis Structure Acetone (C 3 H 6 O) AsCl 3 (Arsenic Trichloride) AsF 3 (Arsenic Trifluoride)




Environment And Climate Change Canada Acts Regulations Ethylene Glycol Final Content




Hydrogen Bonding In Diols And Binary Diol Water Systems Investigated Using Dft Methods Ii Calculated Infrared Oh Stretch Frequencies Force Constants And Nmr Chemical Shifts Correlate With Hydrogen Bond Geometry And Electron Density Topology
Iodate ion (HCO 3) Ion Lewis Structure Bicarbonate ion contains one carbon atom, three oxygen atoms and one hydrogen atom Lewis structure of carbonate ion (HCO 3) contains one C=O bond, two CO bonds and one OH bondThere is 1 charge on one oxygen atom in HCO 3lewis structure HCO 3lewis structure There are three oxygen atoms around center carbon atomLewis Structure 1,4Butanediol (HOCH2CH2CH2CH2OH) Polarity Intermolecular Forces Lewis Structure Hexane (CH3CH2CH2CH2CH2CH) Polarity Intermolecular Forces Lewis Structure Ethanol (CHsCH2OH) Polarity Intermolecular Forces Lewis Structure Acetone (CHsC (O)CH3) Polarity Intermolecular Forces



Chloral Hydrate Cas 302 17 0 Supplier Manufacturer In China India



2



Rcsb Pdb 6tpx N Terminal Bromodomain Of Human Brd4 With 1 1 Acetylpiperidin 4 Yl Methyl 2 4 Hydroxy 3 5 Dimethylphenyl N Methyl 1h Benzo D Imidazole 5 Carboxamide



1



Ethylene Glycol Molecule Of The Month June 18 Jsmol Version



Alcohols 1 Nomenclature And Properties Master Organic Chemistry



2




In The Lewis Dot Structure Of Ethylene Glycol Would One Hydrogen Atom Visibly Connect The Two Oxygen Atoms And If So Would One Line Connecting That Same Hydrogen Atom To One Of



The Chemmaster International Product List Catalog Products
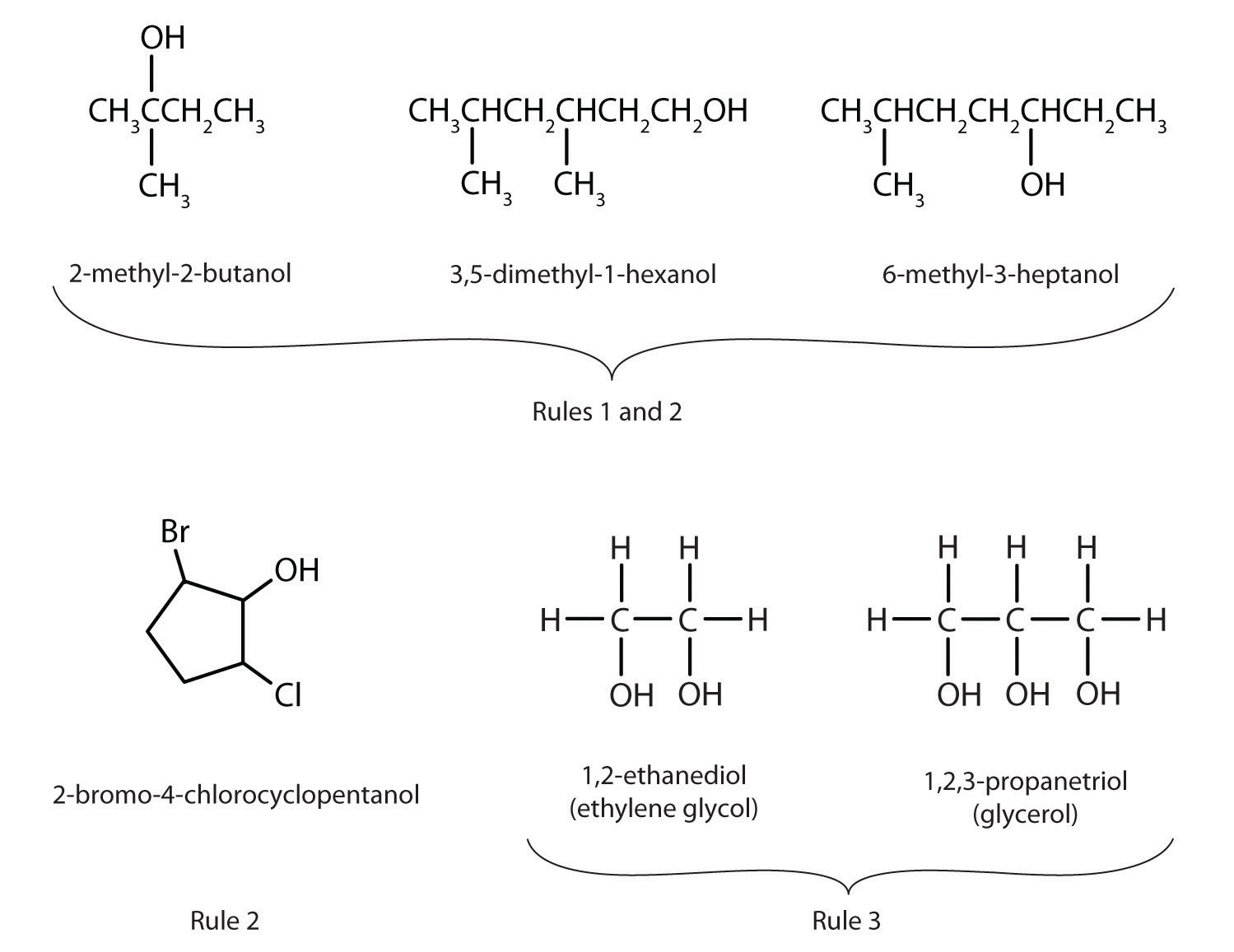



Alcohols Nomenclature And Classification




Solved Change 1 2 Ethanediol Into 1 2 Ethenediol By Removing Chegg Com




Molecule Gallery Alcohols




What Is The Electron Dot Structure Of Ethane Quora
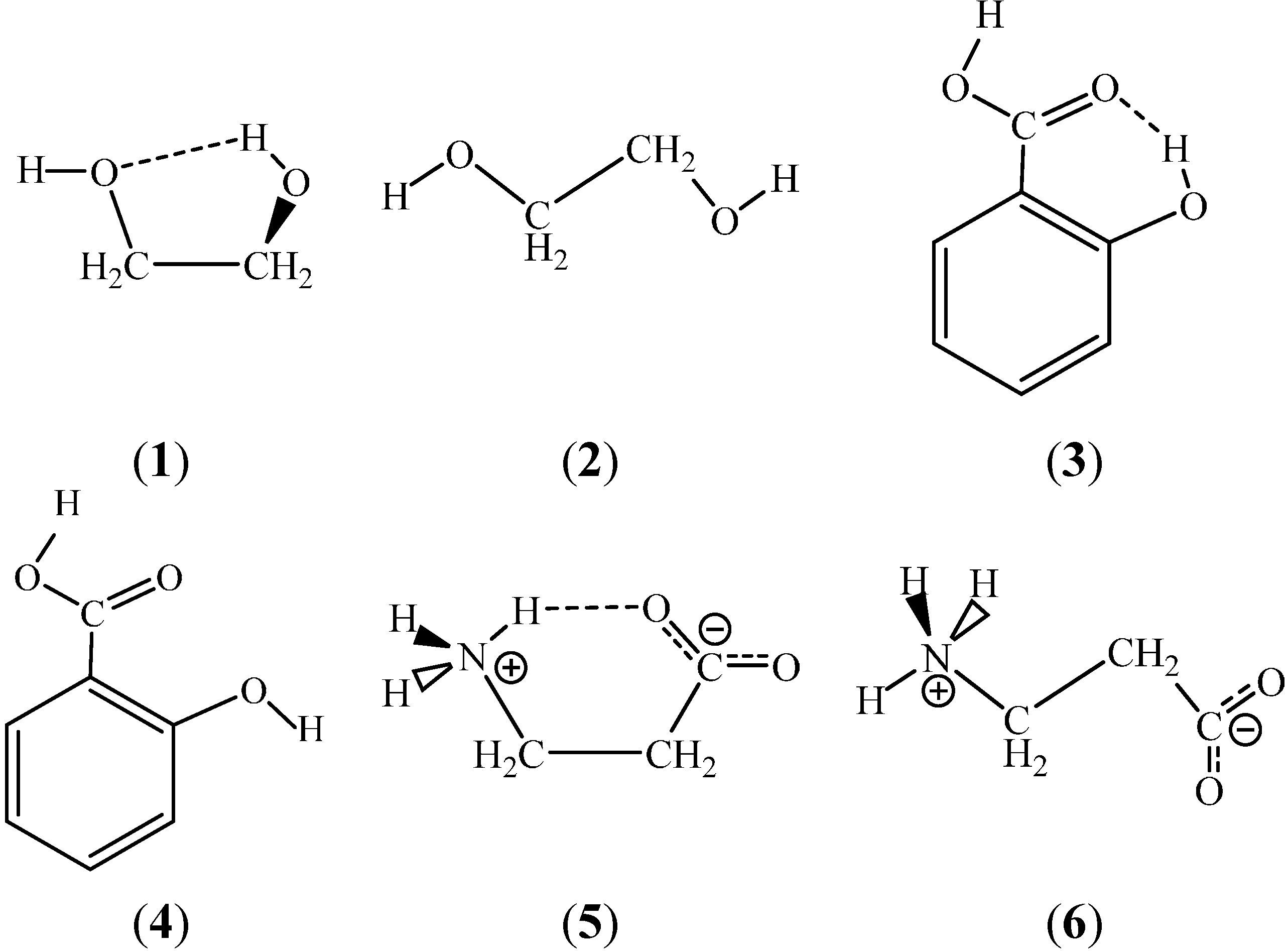



Ijms Free Full Text Competing Intramolecular Vs Intermolecular Hydrogen Bonds In Solution Html
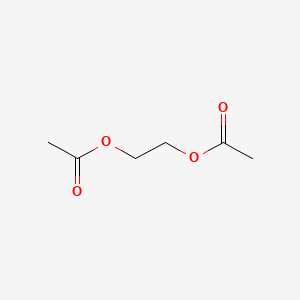



Ethylene Glycol Diacetate C6h10o4 Pubchem



Alcohols And Ethers Chemistry 2e




1 Propanol Lewis Structure Structural Formula Butanol Png Clipart 2butanol Alcohol Angle Area Black Free Png




Organic Chemistry I Homework 1998




Conformational Energies For 1 2 Ethanediol And A D Galactose For Which Download Scientific Diagram
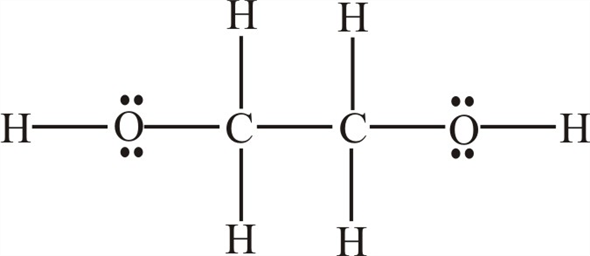



Solved Chapter 8 Problem 116ap Solution Masteringchemistry Standalone Access Card For Fundamentals Of General Organic And Biological Chemistry 7th Edition Chegg Com




Answered 22 What Is The Iupac Name Of The Bartleby
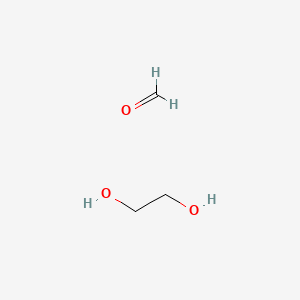



Ethane 1 2 Diol Formaldehyde C3h8o3 Pubchem




Pdbechem Ligand Dictionary Pdb Ligand Chemistry Chemical Component Dictionary




Pushing Curly Arrows



1 2 Ethanediol Structure Shefalitayal
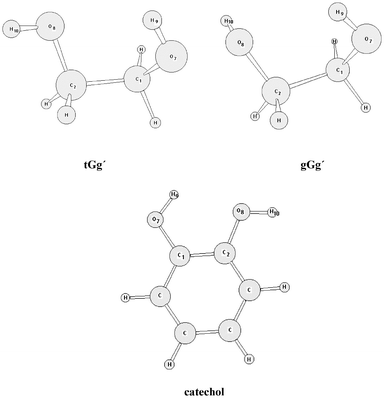



Do 1 2 Ethanediol And 1 2 Dihydroxybenzene Present Intramolecular Hydrogen Bond Physical Chemistry Chemical Physics Rsc Publishing Doi 10 1039 Bc



1




From The Structures Above Select The Comp Clutch Prep
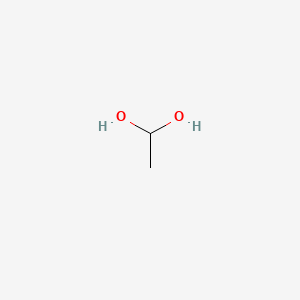



1 1 Ethanediol C2h6o2 Pubchem
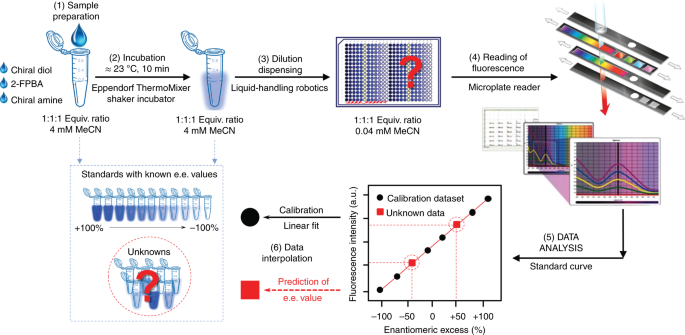



High Throughput Assay For Determining Enantiomeric Excess Of Chiral Diols Amino Alcohols And Amines And For Direct Asymmetric Reaction Screening Nature Protocols




Sch4u Exam Review Questions Glebe
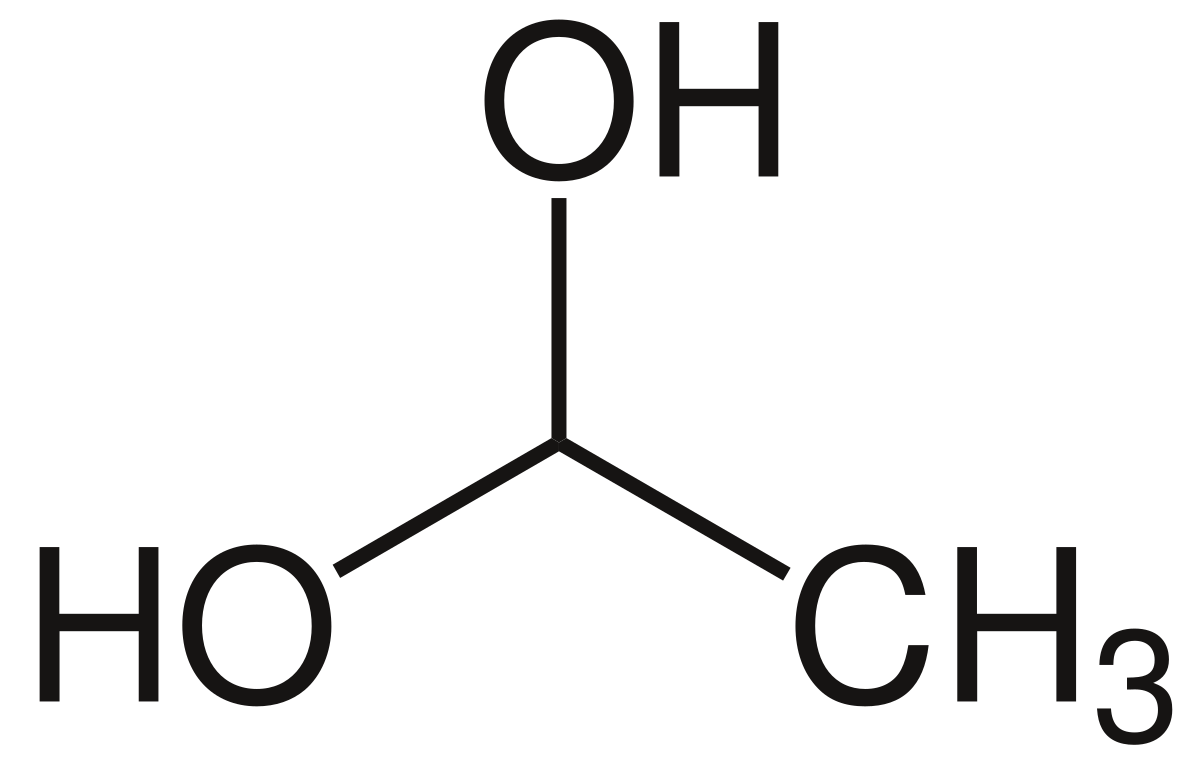



File 1 1 Ethanediol Svg Wikipedia




Molecule Gallery Alcohols



Chemical Product Catalog Letter H Page 265 Chemicalbook



2




542 10 9 Ethylidene Diacetate C6h10o4 Formula Nmr Boiling Point Density Flash Point




Chemistry Homework Help And Exam Questions Page 6245



Ethylene Glycol Molecule Of The Month June 18 Jsmol Version




Efficient Vanadium Catalyzed Aerobic C C Bond Oxidative Cleavage Of Vicinal Diols Amadio 18 Advanced Synthesis Amp Catalysis Wiley Online Library




Draw Both The Condensed And The Structural Formula For Each Of The Following Compounds A 2 2 3 Trimethylpentane B 1 2 Ethanediol C 5 Methyl Trans 2 Heptene Study Com



2



Chloral Hydrate C2h3cl3o2 Chemspider
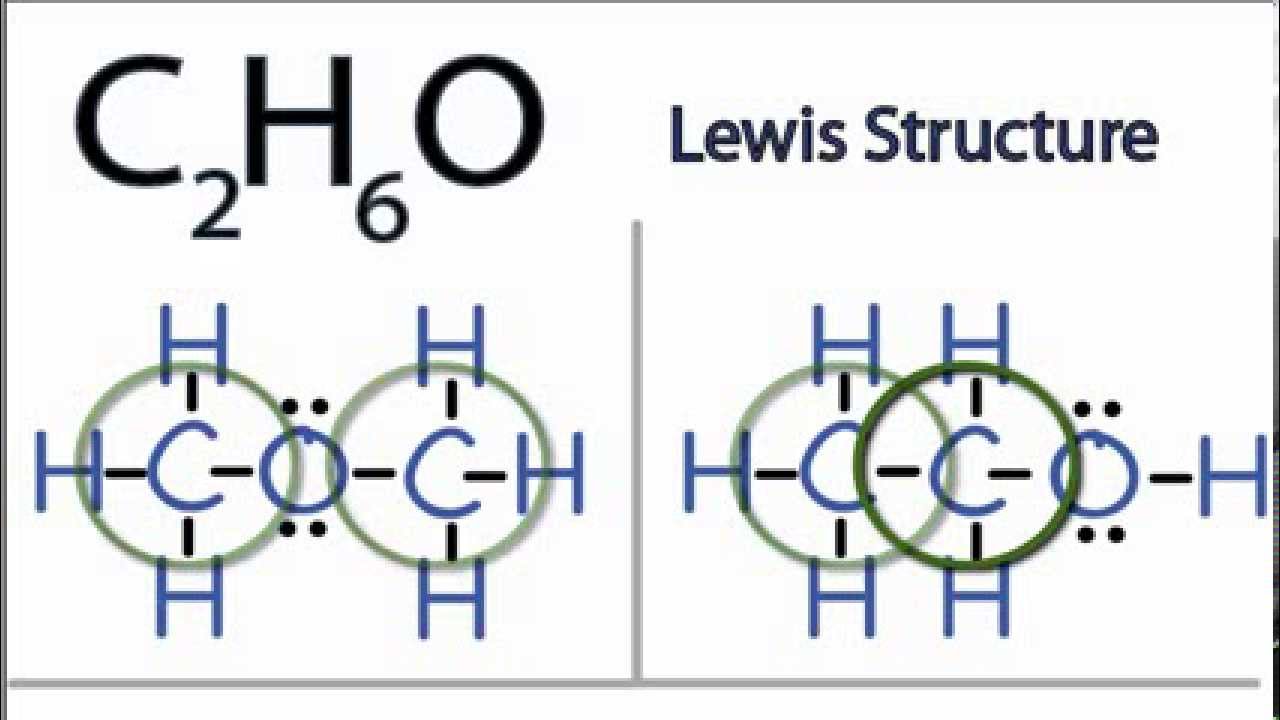



C2h6o Lewis Structure How To Draw The Lewis Structure For C2h6o Youtube




Alcohols And Ethers Chemistry Atoms First




Organic Chemistry




Pdf On The Extent Of Intramolecular Hydrogen Bonding In Gas Phase And Hydrated 1 2 Ethanediol Semantic Scholar



2
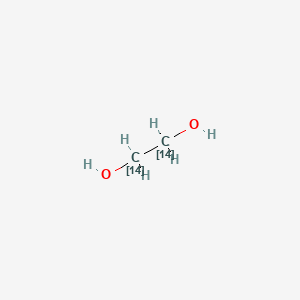



1 2 Ethanediol 1 2 14c2 C2h6o2 Pubchem
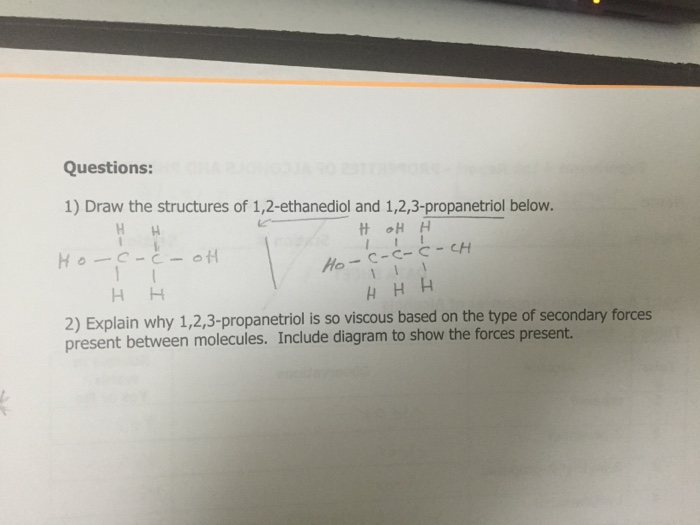



Solved Draw The Structures Of 1 2 Ethanediol And 1 2 Chegg Com



2




61 6 L Threonic Acid Calcium Salt 2r 3s 2 3 4 Trihydroxybutanoic Acid Calcium Salt 2 1 R R S 2 3 4 Trihydroxybutanoic Acid Calcium Salt 2 1 Calcium L Threonate C H O Ca Trc
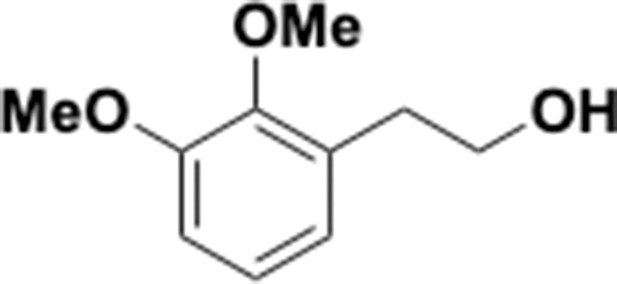



A Widely Distributed Metalloenzyme Class Enables Gut Microbial Metabolism Of Host And Diet Derived Catechols Elife



1 1 Dimethyl 1 2 Ethanediol Molbase
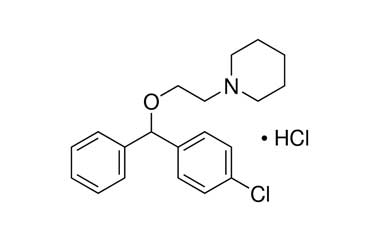



Cloperastine Hydrochloride Impurities Pharmaffiliates




1 1 Ethanediol Structure C2h6o2 Over 100 Million Chemical Compounds Mol Instincts
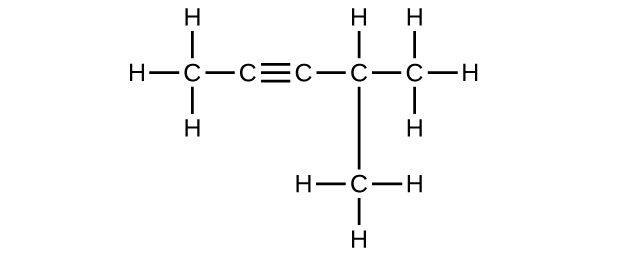



E Organic Chemistry Exercises Chemistry Libretexts




1 Introduction To Organic Chemistry 2 Organic Chemistry Difficult Challenging Memorization Chapter Not Well Maybe Body Of Knowledge Application Ppt Download
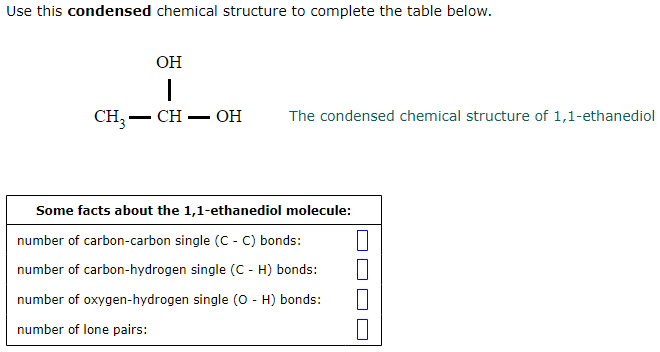



Solved Use This Condensed Chemical Structure To Complete The Chegg Com



1 1 Ethanediol C2h6o2 Chemspider



C2h6 Lewis Structure Etane Hybridization Molecular Geometry And Shape
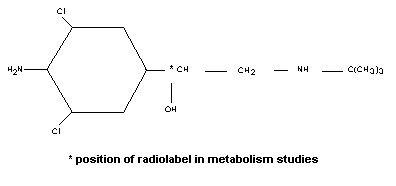



874 Clenbuterol Who Food Additives Series 38
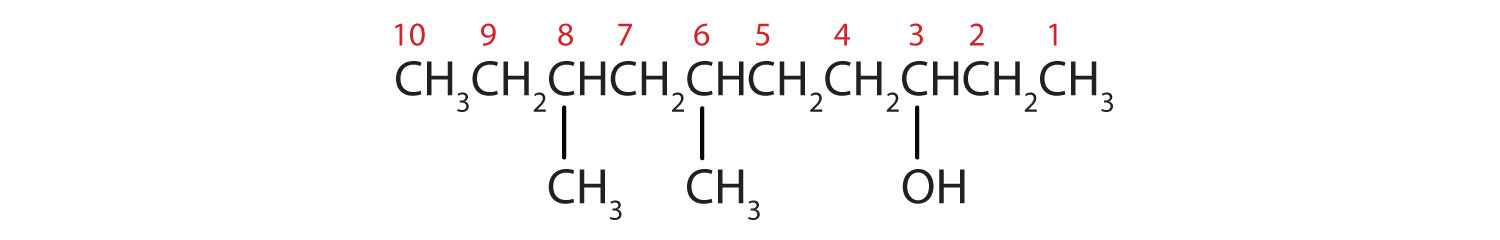



Alcohols Nomenclature And Classification




X Ray Molecular Structure Of Dimim 2 Fe 2 Cl 6 M O 2 Thermal Download Scientific Diagram




Alcohols And Ethers Chemistry Atoms First



2




Pushing Curly Arrows



5dxt P110alpha With Gdc 0326 Rcsb Pdb




Asymmetric Induction Wikipedia



Chapter 2 Alcohols Phenols Thiols Ethers Che 1 Introduction To Organic Chemistry Textbook Libguides At Hostos Community College Library




Acros Organics Ethylene Glycol 99 1l Cas 107 21 1 1 2 Ethanediol 1 2 Ethanediol From Masterflex




Alcohols And Ethers Chemistry Openstax Cnx



Pubs Rsc Org



2
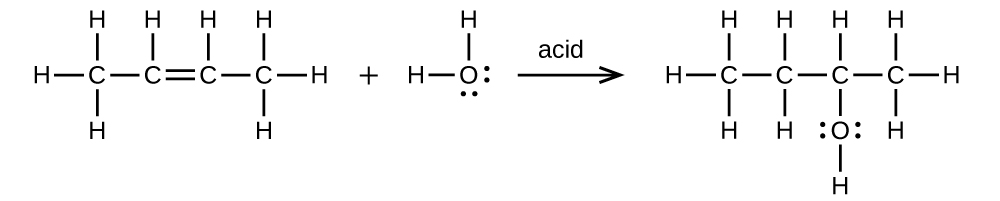



E Organic Chemistry Exercises Chemistry Libretexts




Ch3ch2oh Lewis Structure How To Draw The Lewis Structure For Ch3ch2oh Youtube




Oneclass C Does The Molecule Have Only One Unique Shape In 3 D Hint How Are The Hydrogen Atoms O



1




Ethylene Glycol Detection C2h6o2 Gas Fact Sheet Ion Science Uk




File 1 1 Ethanediol Png Wikimedia Commons




Solved Draw The Electronic Dot Structure Of Ethane Molecule C2h6
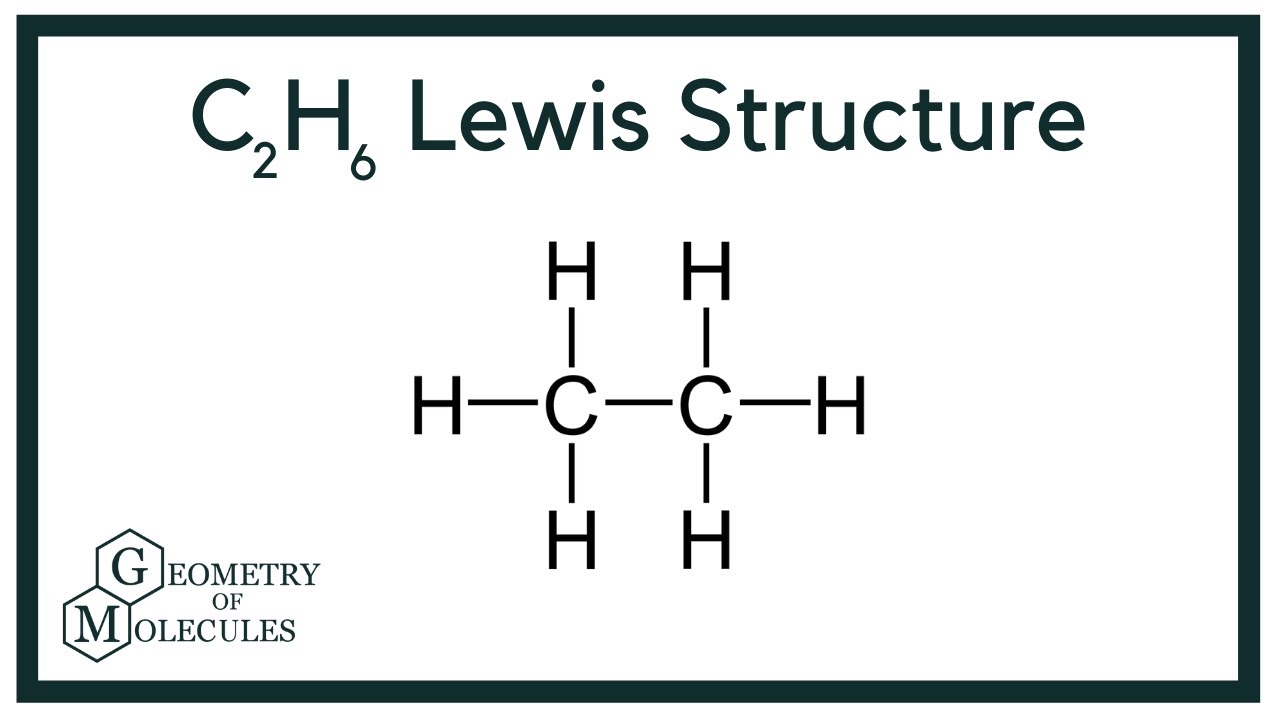



C2h6 Lewis Structure Etane Hybridization Molecular Geometry And Shape




Organic Chemistry And Biochemistry Mcgraw Hill Education Access Engineering



0 件のコメント:
コメントを投稿